what is a chemical bond according to lewis theory
GENERAL Chemical science
COVALENT BOND - LEWIS BONDING THEORY
The sharing of pair of electrons between 2 atoms is referred to as a covalent bond. Normally, each atom that is participating in the covalent bond formation, contributes equal number of electrons to form pair(s) of electrons. The pair of electrons shared between the atoms is as well known every bit bond pair.
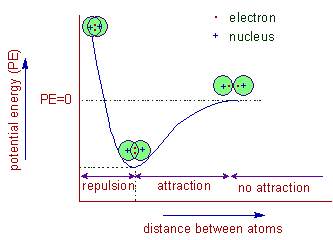
The bond pair is strongly attracted past the nuclei of ii atoms and thus by reducing the potential free energy of them. This is the driving forcefulness of formation of covalent bond, which stabilizes the two atoms.
The distance betwixt the nuclei of two atoms when their potential energy reaches a minima is also known as bail length. The forces of attraction between these two atoms are maximum at this point. However, the repulsion forces dominate over the attraction forces when these atoms are bring farther closer together and thus past increasing the potential energy.
If 2 atoms share only ane bail pair, that bail is referred to every bit a unmarried bail. If ii bond pairs are shared, that is known as a double bond. Also, a triple bail is formed when the atoms share 3 bond pairs.
A covalent bail is formed between two atoms when their electronegativity difference is less than i.7 on Pauling'southward scale. Usually it is formed between two nonmetals.
Polarity of covalent bond: The bond pair is equally shared in between 2 atoms when the electronegativity difference between them is aught or nearer to zero. In this example, neither of the atoms gets backlog of electron density and hence carry no charge. This is chosen not polar covalent bail.

However, when there is a considerable departure in the electronegativity, the bond pair is no longer shared as betwixt the atoms. It is shifted slightly towards the atom with higher electronegativity by creating fractional negative charge (represented by δ-) over it. Whereas, the cantlet with less electronegativity gets partial positive charge (represented by δ+). This blazon of bond is also referred to equally polar covalent bail.

Coordinate covalent bond: Some times, during the germination of covalent bond, the shared pair is entirely contributed by only one cantlet This is called equally coordinate covalent bond or dative bail.
LEWIS DOT MODEL
To explain the formation of covalent bond, a simple qualitative model was developed by Gilbert Newton Lewis in 1916.
According to this model:
* Octet rule: The inert gas atoms with 8 electrons in their outer vanquish (also known as valence shell) are highly stable. The Helium cantlet with 2 electrons in its outer beat is also stable.
Hence every atom tries to get nearest inert gas configuration past sharing electrons. The bail formed due to sharing of electrons is otherwise known every bit a covalent bail.
* Only the electrons in the valence crush are contributed for sharing. The inner electrons, which are also known equally core electrons do not participate in the bail formation.
* In the germination of covalent bond between two atoms, each atom contributes its valence electrons to form pair(due south) of electrons, which in turn is/are shared by both of them.
Due to sharing of electrons, each atom gets nearest inert gas configuration.
Covalency : The number of electrons contributed by the cantlet of an element in the formation of covalent chemical compound is known every bit covalency of that element.
In Lewis dot model, the electrons in the valence shell of the atom are shown as dots around it.
ILLUSTRATIONS OF LEWIS DOT STRUCTURES
1) H2 molecule:
* The electronic configuration of hydrogen is 1s1. Information technology requires one electron to go the configuration of Helium.
* Therefore, during the formation of H2 molecule, each hydrogen atom contributes 1 electron to grade a pair of electrons. This in turn is shared between the ii hydrogen atoms to class a covalent bond.
* Thus in Htwo molecule, each hydrogen atom gets its nearest inert gas: Helium's configuration, 1s2.
* The covalency of hydrogen is 1.
* The Lewis dot structure for Hii molecule is shown below. Note that each hydrogen gets two electrons later on forming the bond. The bond between 2 hydrogen atoms can be shown as a line, which represents a bond pair of electrons.

Annotation: The bond betwixt two hydrogen atoms is not polar since the electronegativity difference is zero.
2) Clii molecule:
* The electronic configuration of Cl is [Ne]3stwo3p5. At that place are 7 electrons in the outer vanquish.
* Therefore, in order to get the nearest inert gas: Argon'south configuration, [Ne]3s23p6, each chlorine cantlet contributes ane electron for the bond formation and form a bond pair, which is shared between the two chlorine atoms.
* Thus Cl2 molecule is formed with a covalent bail betwixt two chlorine atoms.
* In Cltwo molecule, each Cl atom gets 8 electrons in its outer beat out. See the diagram below.
The bond pair is also shown as a line. The electron pairs which do not participate in the bonding are known as lonely pairs. There are three such lone pairs on each of the chlorine atom.
* Hence the covalency of chlorine is i.
* Remember that, in the Lewis dot structures, only the electrons in the valence vanquish are shown.

Annotation: The bond betwixt two chlorine atoms is non polar since the electronegativity difference is nada.
three) Hydrogen chloride (HCl):
* The electronic configuration of hydrogen is 1s1. It requires one electron to get the configuration of He.
* The electronic configuration of chlorine is [Ne]3sii3pv. Information technology as well requires one electron to get the octet configuration.
* Hence, in the formation of HCl molecule, the hydrogen and chlorine atoms contribute one electron each for the bail formation.

Note: The bond betwixt hydrogen and chlorine atoms is considerably polar, since the electronegativity difference betwixt them is iii.five - 2.1 = 1.4. Nonetheless they exercise non form ionic bail since the eastward.due north. difference is less than 1.7.
The hydrogen atom gets fractional positive accuse, whereas the chlorine cantlet gets partial negative charge. However, they are not completely ionized.
4) Methane (CHiv):
* The electronic configuration of carbon is [He]2s22ptwo.
* The electronic configuration of hydrogen is 1si. Hence it contributes this electron to go the nearest inert gas, Helium's configuration.
* The carbon cantlet forms four covalent bonds past contributing four of its valence electrons. Information technology forms 4 bonds with iv hydrogen atoms. Thus it gets octet configuration.
* Covalency of carbon is 4.
* In that location are no lonely pairs on carbon in marsh gas.

Notation: The electronegativity departure between carbon (e.n. = two.5) and hydrogen (e.n. = 2.1) is not considerable. Hence the covalent bail formed is practically considered as non polar.
v) Ammonia (NH3) :
* The electronic configuration of nitrogen is [He]2s22p3. Nitrogen require 3 electrons to consummate the octet.
* The electronic configuration of hydrogen is 1s1 and hence in need of one electron to complete the shell.
* In the formation of Ammonia molecule, the nitrogen atom contributes 3 of its valence electrons to form three bond pairs which are shared with hydrogen atoms. Thus nitrogen forms 3 single bonds with three hydrogen atoms and gets the configuration of Neon.
* At that place is also one solitary pair on nitrogen atom.
* Covalency of nitrogen is 3.

Note: There is considerable polarity in N-H bond since the electronegativity difference between nitrogen (e.north. = 3.0) and hydrogen (e.n. = 2.1) is 0.nine.
Being highly electronegative than hydrogen, the nitrogen atom gets partial negative charge, whereas the hydrogen atom gets fractional positive charge.
half-dozen) H2O molecule :
* The electronic configuration of oxygen is [He]2stwo2p4. Hence it requires 2 more electrons to consummate its octet.
* In the germination of water molecule, the oxygen atom contributes two of its valence electrons to form two bond pairs that are shared with ii hydrogen atoms separately. Thus ii bonds are formed past oxygen atom to go the configuration of Neon.
* There are also two lone pairs on oxygen atom.
* Covalency of oxygen is two.

Note: The O-H bail is besides considerably polar since the electronegativity difference between oxygen (e.n. = 3.5) and hydrogen (e.due north. = two.1) is ane.4.
Since the oxygen cantlet is more than electronegative, information technology gets partial negative charge.
7) Dioxygen molecule (O2):
* In the germination of dioxygen molecule, each oxygen atom contributes 2 electrons to form 2 bail pairs, which are shared by the two oxygen atoms. Thus each oxygen cantlet gets Neon's configuration.
* At that place is a double bond between two oxygen atoms.
* Notwithstanding there are two lone pairs on each oxygen cantlet.

8) Dinitrogen molecule (N2):
* In the germination of Dinitrogen molecule, each nitrogen atom contributes 3 electrons to grade 3 bond pairs, which in turn are shared by 2 nitrogen atoms. Each nitrogen cantlet gets Neon's configuration.
* Thus a triple bond between nitrogen atoms is formed.
* Each nitrogen also contains ane lonely pair.
![]()
ix) Carbon dioxide molecule (CO2):
* The carbon atom contributes four of its valence electrons, whereas each oxygen atom contributes two electrons for the bond formation to complete their octets.
* There are two electron pairs shared between carbon and 1 of the oxygen atom i.e., a double bail, C=O is formed. In that location are two such C=O bonds in CO2 molecule.
![]()
Note: The C=O bond is polar due to electronegativity difference between carbon (e.n. = 2.i) and oxygen (e.n. = 3.5) is 1.four.
Question to ponder: What is the overall polarity of the carbon dioxide molecule? Is information technology polar or not polar?
Molecules violating octet dominion:
1) BeClii (Beryllium chloride):
* The electronic configuration of Beryllium is 1s22s2.
* The electronic configuration of Chlorine is [Ne]3s23p5.
* During the formation of Beryllium chloride, the glucinium atom contributes its two valence electrons and forms two bond pairs. These are shared with chlorine atoms.
* In BeCl2 in that location are merely four electrons in the valence trounce of Be. However the Beryllium chloride is a relatively stable molecule. This is the violation of octet rule.
* Covalency of beryllium is 2.

Note: The beryllium atom may lose 2 of its valence electrons to become the nearest inert gas: Helium'south configuration and form Be2+ ion. However, the electronegativity difference between Glucinium (east.northward. = ane.5) and that of chlorine (e.n. = three.0) is less than 1.7. Hence the bond between them has considerable covalent character rather than the ionic nature.
two) BCl3 (Boron trichloride):
* The electronic configuration of Boron is 1s22s22pane.
* During the formation of BCl3 molecule, the boron cantlet contributes three of its valence electrons to form three bond pairs with chlorine atoms.
* Thus there are only six electrons in the valence beat of boron cantlet in BClthree. Still, still this molecule is stable. It is an electron deficient compound. Information technology is likewise the violation of the octet rule.
* Covalency of boron is 3.

Notation: The bail between boron (e.n. = 2.0) and chlorine (e.n. = 3.0) is covalent since the electronegativity difference between them is less than 1.7.
iii) PCl5 (Phosphorous pentachloride):
* Electronic configuration of phosphorus is [Ne]3s23p3.
* Electronic configuration of chlorine is [Ne]3stwo3pv.
* In the germination of PCl5 molecule, phosphorus contributes five electrons in it's valence shell and forms 5 bonds with chlorine atoms.
* Thus there are 10 electrons in the valence vanquish of phosphorus in this molecule. It is a stable molecule, yet, violating the octet rule.
* Covalency of phosphorus in this molecule is five.

4) SF6 (Sulphur hexafluoride) :
* The electronic configuration of Sulphur is [Ne]3s23p4.
* The electronic configuration of Fluorine is [He]2s22p5.
* The fluorine cantlet requires one electron to complete its octet. Hence it contributes one electron for bonding.
* The Sulphur atom contributes vi of its valence electrons to form 6 bonds with half dozen fluorine atoms. Thus there are 12 electrons in the valence shell in sulphur atom.
* The SFsix molecule is quite stable even though it violates the octet dominion.
* Covalency of sulphur in this molecule is 6.

DRAWBACKS OF LEWIS THEORY
* Lewis theory could non explicate the geometry of molecules and bond angle in them.
* Information technology could not explain why some molecules are violating the octet rule.
* This is a qualitative explanation for covalent bond only.
To fulfill these gaps and to explain the covalent bond formation quantitatively, the Valence bond theory (VBT) was put forwarded. You can find more insight into this theory in next section.
However it is important to learn VSEPR theory, another qualitative model, which was put forwarded to explain the shapes of molecules, before moving on to Valence bail theory.
By Aditya vardhan VutturiSource: https://www.adichemistry.com/general/chemicalbond/covalentbond/covalent-bond.html
Post a Comment for "what is a chemical bond according to lewis theory"